Pure Substance: Unveiling the True Nature of Matter
Pure Substance: Unveiling the True Nature of Matter
Introduction
Matter is more complex than it appears at first glance. Everyday items such as milk, salt, and spices may be labeled as "pure," but scientific inquiry tells us that most of these items are mixtures. In this post, we dive deep into the science behind pure substances, exploring their true nature, and examining how mixtures, solutions, suspensions, and colloids form the foundation of our material world.
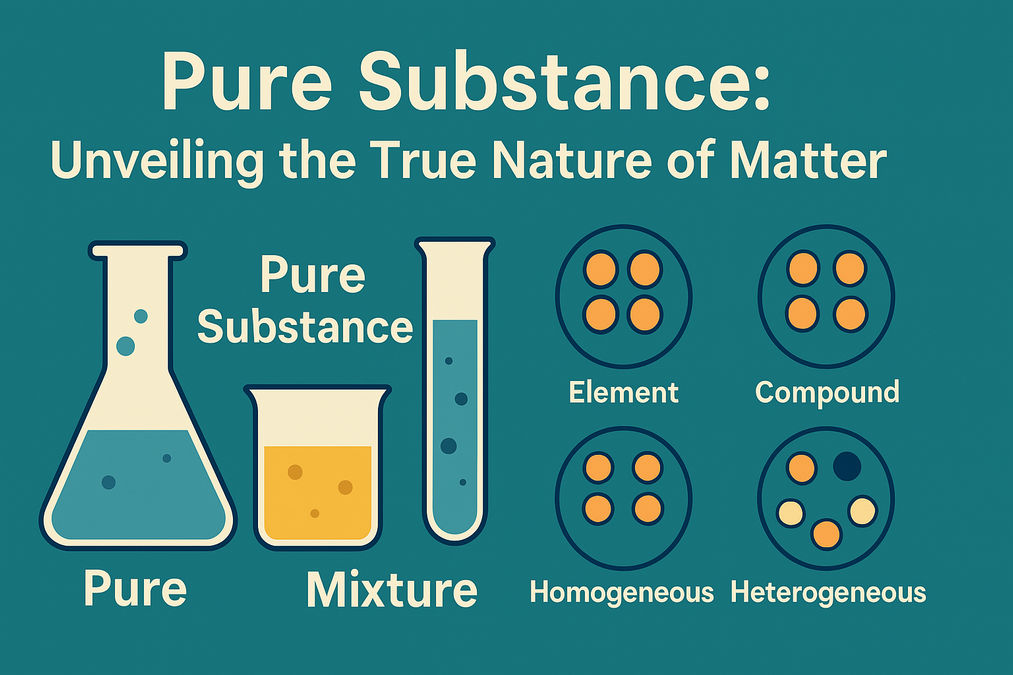
What Is a Pure Substance?
A pure substance is a form of matter that contains only one type of particle. Whether it’s an element like oxygen and iron or a compound like water, these substances maintain a uniform composition throughout. Unlike mixtures, where various components combine physically, pure substances always display the same chemical properties no matter where or how you sample them.
Characteristics of a Pure Substance
- Uniform Composition – every sample has the same chemical properties.
- Fixed Composition – the internal ratio of components is set.
- Non-Separability by Physical Means – only chemical methods can break it down into its constituent components.
Understanding Mixtures: Homogeneous & Heterogeneous
Most of the matter we observe daily is not pure but a mixture. A mixture combines two or more substances—each of which is a pure substance—and can be separated by physical processes. Mixtures can be classified into:
Homogeneous Mixtures (Solutions)
Homogeneous mixtures, or solutions, have particles distributed uniformly at a molecular level. In these mixtures, even though the concentration can vary (for example, two copper sulphate solutions of different intensities), the appearance remains consistent. Common examples include sugar dissolved in water and well-blended teas.
Heterogeneous Mixtures
Heterogeneous mixtures contain visibly distinct parts. These mixtures can be further subdivided:
- Suspensions: Particles are large enough to be seen and eventually settle – like mud in water.
- Colloids: Small particles that remain dispersed and scatter light, such as milk, which exhibits the Tyndall effect.
Exploring Solutions, Suspensions, and Colloids
What Is a Solution?
A solution is a homogeneous mixture formed when a solute dissolves uniformly in a solvent. Common examples include sugar solutions, carbonated beverages, and even solid solutions like brass. The particles in a solution are extremely small – typically less than 1 nm – making them undetectable by the naked eye.
Concentration and Solubility
The concentration of a solution refers to the amount of solute present in a given quantity of solvent and is often expressed as mass by mass, mass by volume, or volume by volume percentage. When a solution holds the maximum amount of solute possible at a given temperature, it is considered saturated. Changes in temperature can significantly affect how much solute dissolves.
Suspensions
Suspensions are heterogeneous mixtures where larger particles are dispersed in a liquid and may settle over time. The visible particles allow for easy separation using processes like filtration.
Colloids
Colloids represent a unique state of matter with particles that are intermediate in size between those in solutions and suspensions. Though too small to see with the naked eye, they are large enough to scatter light. This scattering, known as the Tyndall effect, is clearly demonstrated by common examples such as milk and fog.
Physical and Chemical Changes in Matter
Physical Changes
Physical changes affect only the state or appearance of a substance without altering its chemical makeup. Examples include processes like melting, freezing, or dissolving—where the substance's physical state changes but its chemical formula remains constant.
Chemical Changes
In contrast, chemical changes result in transformations that create entirely new substances with different properties. The burning of paper, rusting of iron, or cooking of food are prime examples where the chemical structure changes, producing compounds with new characteristics.
Elements vs. Compounds: Classifying Pure Substances
Pure substances are divided into two primary categories: elements and compounds. Elements are the simplest forms of matter that can’t be broken down into simpler substances by chemical reactions (like oxygen, iron, or gold). Compounds, however, are substances created when two or more elements chemically combine in a fixed ratio, forming a new substance with properties distinct from its constituents – water being the most common example.
Separation Techniques: Unmixing the Mixture
Recognizing whether matter is a pure substance or a mixture is significant not only in theory but also in practical scenarios. Scientists and learners use several separation techniques:
- Filtration: Effective for suspensions where larger particles settle out.
- Evaporation: Allows recovery of a solute by removing the solvent.
- Centrifugation: Separates colloidal particles by spinning at high speeds.
- Chemical Reactions: Required for decomposing compounds into their elemental forms.
Key Experiments and Classroom Activities
Many classroom experiments illustrate the concepts of pure substances versus mixtures. For example, mixing copper sulphate with varying amounts of water can demonstrate the difference in concentration of solutions. Using filtration to observe particle settling in suspensions, or shining a beam of light through colloids to visualize the Tyndall effect, offers practical insight into these scientific principles. Additionally, experiments combining iron filings and sulphur—showing both physical and chemical changes—help reinforce why a thorough understanding of matter is fundamental.
Conclusion
In conclusion, exploring the realm of pure substances deepens our understanding of the material world. Although many items labeled as pure may seem simple, a closer examination reveals an interplay of mixtures, solutions, suspensions, and colloids. Grasping these differences is vital for both scientific research and everyday applications, providing essential knowledge that underpins various fields—from chemistry and engineering to quality control in manufacturing.
Whether you are a student, educator, or science enthusiast, understanding these concepts enriches your appreciation for the complexity of matter. As scientific inquiry continues to evolve, every discovery about the nature of pure substances opens up new opportunities for innovation and exploration.
Call to Action
Loved this detailed exploration into the nature of matter? Subscribe to our blog for more scientific insights, in-depth experiments, and practical tips that will enhance your understanding of chemistry and everyday science. Join our community today to start your journey toward mastering the fascinating world of pure substances!
📲 Get Instant Updates
Want Sarkari Job Alerts, Exam Dates, Notes & PDF? Join our official WhatsApp Channel now!
Get Daily Govt Jobs Alerts, Notes & Updates!
Join the conversation